Translate this page into:
An unusual case of incomplete healing with intraosseous fibrous scar formation in the surgically enucleated site associated with a Type II dens invaginatus: A 4-year follow-up case report

*Corresponding author: Ruchi Shah, Department of Conservative Dentistry and Endodontics, K. M. Shah Dental College and Hospital, Sumandeep Vidhyapeeth, Vadodara, Gujarat, India. ruh006@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shah R, Pant Y. An Unusual case of incomplete healing with Intraosseous Fibrous scar formation in the surgically enucleated site associated with a Type II dens invaginatus: A 4-year follow-up case report. J Restor Dent Endod 2021;1(1):23-9.
Abstract
Dens invaginatus is a developmental deformation with varying anatomical features, caused by the envelopment of the enamel organ and/or the Hertwig’s epithelial root sheath within the tooth before calcification completes, increasing the vulnerability of pulpal and periodontal inflammation, posing challenges to treatment, and adequate healing. Cell rest of Malassez, remnants of enamel organ or root sheet may cause cystic lesion formations, also hinder the normal healing process and form a fibrous scar. Intraosseous fibrous scar is a result of incomplete wound healing after periradicular surgery which mimics an asymptomatic residual cyst clinically and radiographically. We herein report a case of a surgically managed an immature maxillary lateral incisor affected with Type II dens invaginatus associated with incomplete periradicular healing presented as intraosseous fibrous scar with a persistent well-defined radiolucency between the healthy trabecular bone. The regular clinical and radiographic follow-up records the asymptomatic endodontically treated dens invaginatus with an intact lamina dura and regular healing trabecular bone pattern. Its 4 years of post-operative evaluation has been presented. Features of this case, causes of such incomplete healing, diagnosis, treatment line, prognosis, and the dilemma of the clinician are discussed together with its probable implications.
Keywords
Dens invaginatus Type II
Incomplete healing
Intraosseous fibrous scar
ProRoot mineral trioxide aggregate
Surgical endodontic treatment
INTRODUCTION
Dens invaginatus is a tooth developmental abnormality resulted from an infolding of the outer surface of the teeth into its interior. It can occur in the crown or root during tooth formation and may involve the pulp chamber or root canal, which may results in deformity in the crown or the root. The extreme form of dens invaginatus is called as dilated odontome.[1,2]
Ridell et al. reported that most commonly found dens invaginatus type is Type I which is around 79% in which the infolding is within the crown, followed by Type II that is 15% in which the infolding extends into the pulp chamber but remains within the root canal does not communicate with the periodontal ligament and Type III is least common that is 5% in which the infolding perforates the root communicating with the periodontium (Oehlers’ classification, 2008).[3] In a study North Indian population, 0.55% prevalence of dens invaginatus has been shown of which 88.3% were in lateral incisor.[4]
It is quite common to find dens invaginatus cases, but it is often missed clinically due to the absence of any significant clinical signs and symptoms except for Type III. This missed condition of the tooth is regrettable as the presence of an infolding of enamel and dentin is considered to increase the risk of caries, pulpal pathosis, and periodontal inflammation.[1]
Many theories have been proposed for its etiology. Some authors suggested that genetic influence plays a major role in such anomalies. One probable reason may be an ingrowth of the enamel organ or Hertwig’s epithelial root sheath within the dental papilla.[2]
The remains of the enamel organ and the root sheath are represented by cell rest of Malassez in the periodontal ligament, which is also seen in dens invaginatus cases. These cells proliferation in periodontal tissues are known to be involved in several dental pathologies such as the formation of a developmental cyst, gingival or lateral periodontal cyst paradental, and peri-apical cysts.[5]
It was hypothesized in 2007 by Lin et al. that aggregation of epithelial strands of cell rest of Malassez present in the periodontal tissue leads to the formation of inflammatory apical cysts. The inflammatory mediators mediated by cell rest of Malassez involved in the formation of an inflammatory cyst, further causes hindrance in periapical healing process by mediating bone resorption.[6]
After surgical or non-surgical endodontic procedures, periapical healing process should advance with regeneration, in which the healed tissues normal function and microarchitecture are restored, rather than repair, which is a fibro-proliferative response of the body. Repair results in the formation of a typical fibrous scar tissue, thus the tissues normal architecture and function are not restored.[7,8]
Radiographic, clinical, and histological assessment helps to determine whether healing is adequate and can be ascertained to remain asymptomatic without any inflammation or whether the healing status is debatable, or if the treatment is not successful and require resurgical intervention and extraction of the tooth involved (Andreasen, 1972). For evaluating this, status patient support as follow-up visits for a long duration is required.[9]
The gold standard for evaluation of the periapical healing status remains the histologic examination, which gives the most precise post-operative periapical healing estimation in both the surgical and conventional endodontic therapy. Unfortunately, it is not feasible to use this method clinically on every patient, and thus the results are assessed and evaluated by a clinical and a radiographic examination approach. A periradicular radiolucency seen between the trabecular healing bone and above the healed periodontium of the involved tooth at multiple follow-ups visits may constitute a typical bone regeneration phase or due to incomplete healing with the formation of an intrabony fibrous scar tissue or is a failure of treatment caused by inflammatory residues formed due to insufficient enucleation and debridement.[10,11] Hence, a state of the dilemma for clinician remains whether to opt for resurgical intervention or wait for further time being.
Serman mentioned the similarity between residual dental cyst radiographically with the intraosseous fibrous scar. Radiographically an intraosseous fibrous scar and a residual cyst, both, presents as a round, unilocular radiolucency with distinct borders making it difficult to differentiate between them radiographically. The only difference being the site of formation, residual cyst is known to occur in an edentulous area only.[12]
To author’s best knowledge, no case has been reported regarding the intraosseous fibrous scar formation associated with dens invaginatus Type II.
Hence, the objective of this publication is the presentation of a clinical case of intraosseous fibrous scar associated with surgically enucleated large radicular cyst with maxillary lateral incisor affected by Type II dens-invaginatus and its 4-year follow-up. The dilemma, the probable causes, diagnosis, treatment line, prognosis, and its implications are further described.
CASE REPORT
A 36-year male patient came to our department for a chief complaint of swelling in the palatal region and associated pain with left upper front tooth region and generalized stains on all his teeth. Patient has a history of trauma in his childhood and tobacco chewing habit since 5–6 years. Patient gives family history of stains in all his family members. No relevant associated medical history present.
On oral examination, there was a swelling of 2.5 cms × 2 cms diameter present along the palatal aspect of 21, 22, 23, and 24 medially extending toward the mid-palatine raphe [Figure 1a]. The swelling was soft and fluctuant, easily compressible without tenderness.
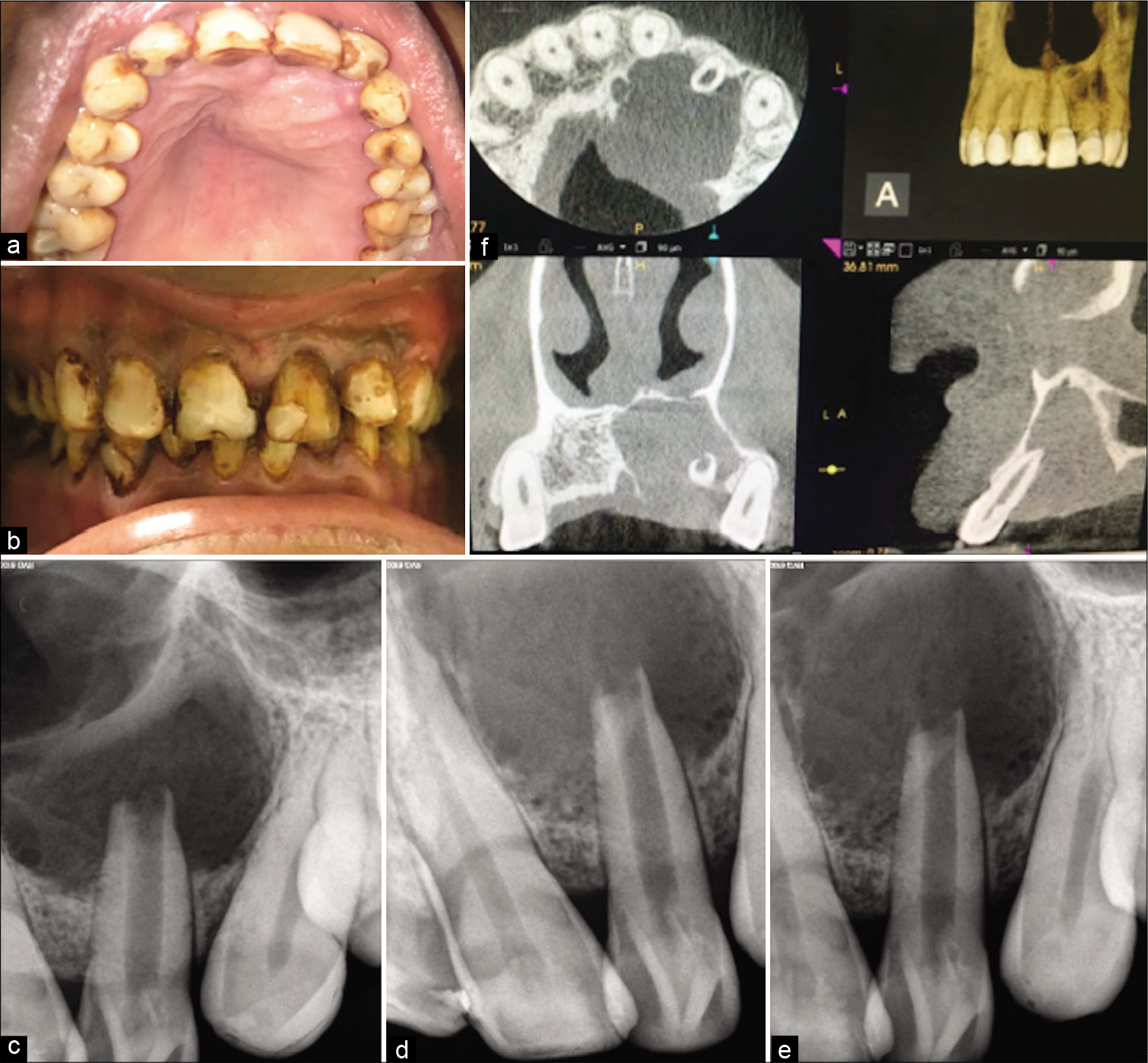
- (a) Intra-oral clinical picture of the maxillary arch showing the unilateral round palatal cystic swelling, sinus opening associated with left lateral incisor and canine, and presence of atypical cingulum and protuberance with both the lateral incisors (b) Frontally centered intra-oral photograph showing buccally protruded lateral incisor and generalized moderate to severe fluorosis stains (c) front angulated pre-operative radiograph showing the large lesion associated with lateral incisor (d) mesial angulated pre-operative radiograph showing the Type II dens invaginatus with lateral incisor infolding into the pulp canal (e) distal angulated pre-operative radiograph showing the Type II dens invaginatus with lateral incisor infolding into the pulp canal (f) pre-operative cone-beam computed tomography image with all the sections. Axial image showing the extent of destruction of the palatal bone. Frontal section showing the involvement of inferior nasal wall along with the palatal cortical plate. Sagittal section showing the infolding of dentin into the pulp canal space with lateral incisor along with the palatal bone involvement.
On further examination, 22 were buccally protruded, were tender to percuss, and had a prominent cingulum and an associated sinus opening on the palatal surface. Teeth had generalized moderate to severe stains, were non-mobile with normal probing depths [Figure 1b]. On radiographic examination, a large diffuse radiolucency seen associated with 21, 22, and 23. From three different angulations radiographs were taken, with mesial and distal angulated radiographs radio-opaque dentin infolding was much evident with 22 which was invading into the pulp chamber and canal suggestive of Type II dens-invaginatus [Figures 1c-e]. Open apex and blunderbuss canal were associated with 22 suggestive of incomplete root formation associated with dens invaginatus anomaly. On pulp sensibility testing 21 and 22 showed a negative response while 23 showed a delayed response. Cone-beam computed tomography (CBCT) of the arch was suggested due to the extent of lesion suspected. With the help of CBCT exact peripheries of the cyst, extensions were assessed which was along the two teeth, that is, 21, 22, and slight involvement of 23. A complete destruction of palatal cortical plate and perforation of the inferior nasal floor was evident. With the respect to tooth 22 radio-opaque dentin mass is seen invading into the pulp canal in a coronal section of CBCT [Figure 1f]. Hence, a diagnosis of large radicular cyst associated with the necrotised pulp with tooth 22 associated with type II dens invaginatus was provisionalized and a non-surgical endodontic treatment plan was first proposed to the patient.
The treatment protocol, the prognosis was explained to the patient and was accepted by the patient. Access opening of 21, 22, and 23 were performed under proper isolation [Figure 2a]. Working length was determined. Pus drainage was established by extending the no. 20 K file 2 mm beyond the apex [Figure 2b]. Canal was thoroughly rinsed with saline, 17% EDTA and 2% chlorhexidine solution in between. The patient was asked to wait for 30–45 min until pus drainage occurs. After complete drying of the canal, calcium hydroxide dressing was given 2 times in 7 days interval and triple antibiotic paste was given. However, unfortunately, the patient returned with swelling every time and draining canal with tooth 22 hindering the option of obturation with tooth 22. While tooth 21 and 23 were not affected and canals were completely dry. Hence, surgical removal of the cyst was opted before obturation with tooth 22, and obturation of 21 and 23 was performed. As critical areas of the palate and inferior nasal floor were at risk, cyst enucleation under GA was suggested by the oral and maxillofacial surgeons.
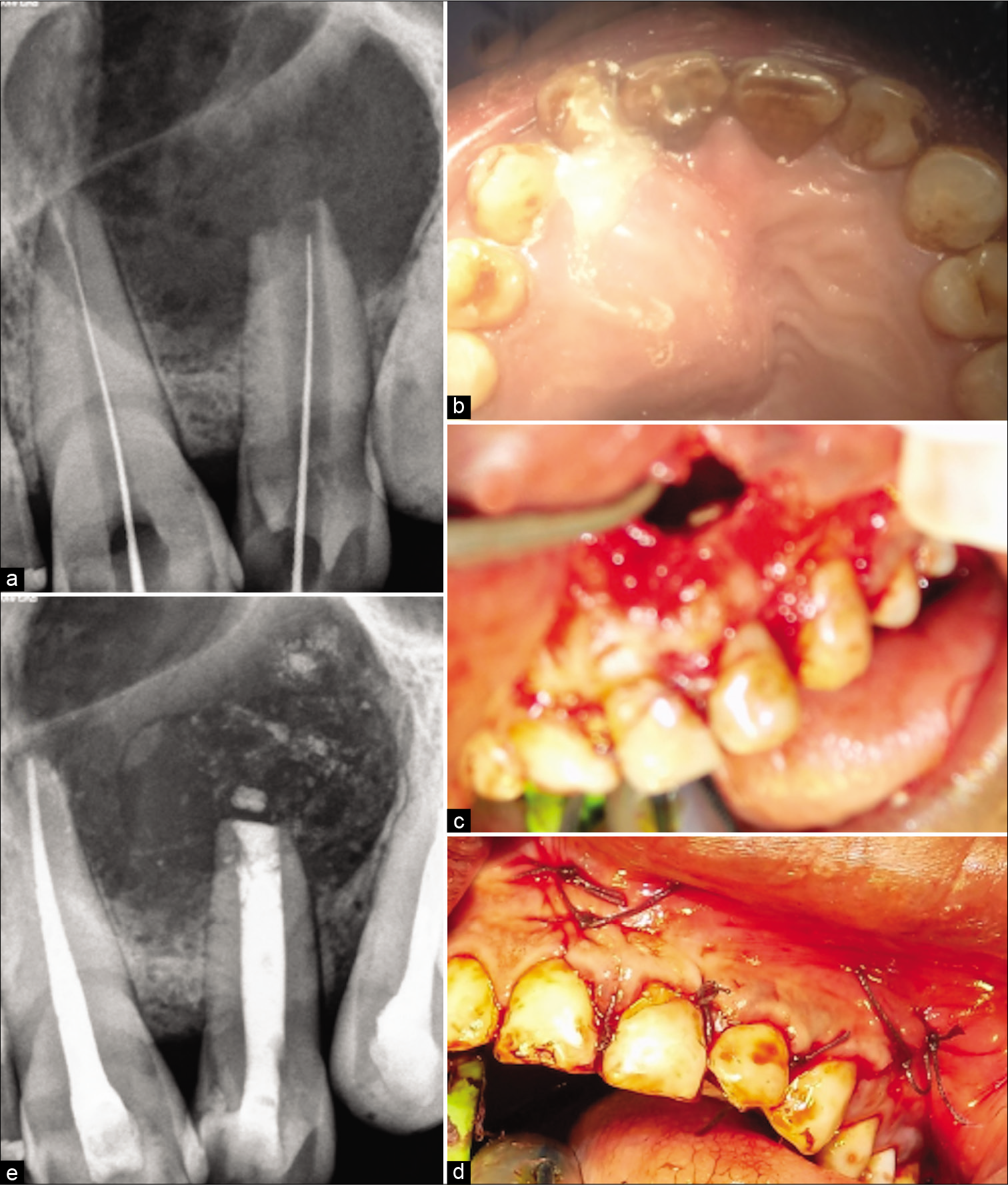
- (a) Radiograph showing the working length determination and creating a pathway from pus drainage for the periapical exudates (b) Clinical intra-oral upper occlusal picture showing the drainage of exudates of the cystic swelling through the lateral incisor affected with type II dens invaginatus (c) clinical image after the flap reflection and osseous window creation showing the root apex after root resection for further retrograde mineral trioxide aggregate (MTA) filling (d) clinical picture with post-operative sutures (e) post-operative radiograph showing retrograde 3 mm MTA plug and orthograde thermoplastized obturation with 22 and lateral compacted 21. Note the lesion size in this radiograph.
Under GA Flap was raised, an osseous window was created, the complete cyst was enucleated and sent for histological examination, root apex was located, the site was rinsed thoroughly with beta-dine. As the apex was accessible, tooth was thoroughly irrigated and cleaned [Figure 2c]. Considering the length and development of root 1.5 mm of the root was resected and 3 mm retrograde Mineral Trioxide Aggregate (MTA) (ProRoot® MTA, DENTSPLY) was restored. Sutured were placed [Figure 2d]. Patient was prescribed antibiotics for 5 days and NSAIDS for 7 days as and when needed. Patient was recalled after 3 days for obturation. Histological examination reported an inflammatory radicular cyst confirming our provisional diagnosis. The patient was completely asymptomatic; hence, thermoplastic obturation was performed with 22 and laterally condensed obturation with 21 and 23 [Figure 2e]. Post-operative radiographs were taken and the patient was asked for scheduled follow-ups. Patient came for regular follow-ups and radiographic evaluations in 3 months [Figure 3a], 6 months [Figure 3b], 1 year [Figure 3c], 2 years [Figure 3d], 3 years [Figure 3e], and 4 years [Figure 3f] follow-up. After 1 year, the patient was completely asymptomatic and trabecular bone healing was seen along with small well-described radiolucency. As patient was completely asymptomatic and hence was kept on follow-up, further esthetic treatments were initiated. The patient was aesthetically compromised and was willing for esthetic rehabilitation. Considering the economic status, severity of fluorosis and age of the patient, full jacket crown was suggested as a treatment option which was accepted by the patient [Figure 4]. After 4 years of radiographic assessment, lamina dura of all three teeth was nearly intact and a trabecular healing pattern was visible in each radiograph, but 2–3 mm above the root apex of lateral incisor affected with Type II dens invaginatus a well-defined radiolucent area with demarcated peripheries was still present which was a reason of worry for both patient and clinician. Hence, an expert oral surgeon was called for his opinion. Fine-needle aspiration cytology (FNAC) and histological examination were the only option to rule out the possibility of any inflammation or residual cyst formation. During inspection and palpation, no tenderness and soft-tissue swelling was evident due to complete bone healing around the lesion. Patient was explained about CBCT guided FNAC procedure or excision as last resort to rule out the possibility of inflammation, if any symptoms are present. As the patient was completely asymptomatic, the diagnosis of incomplete healing with intraosseous scar formation was provisionalized and he is kept on further follow-ups for the same.
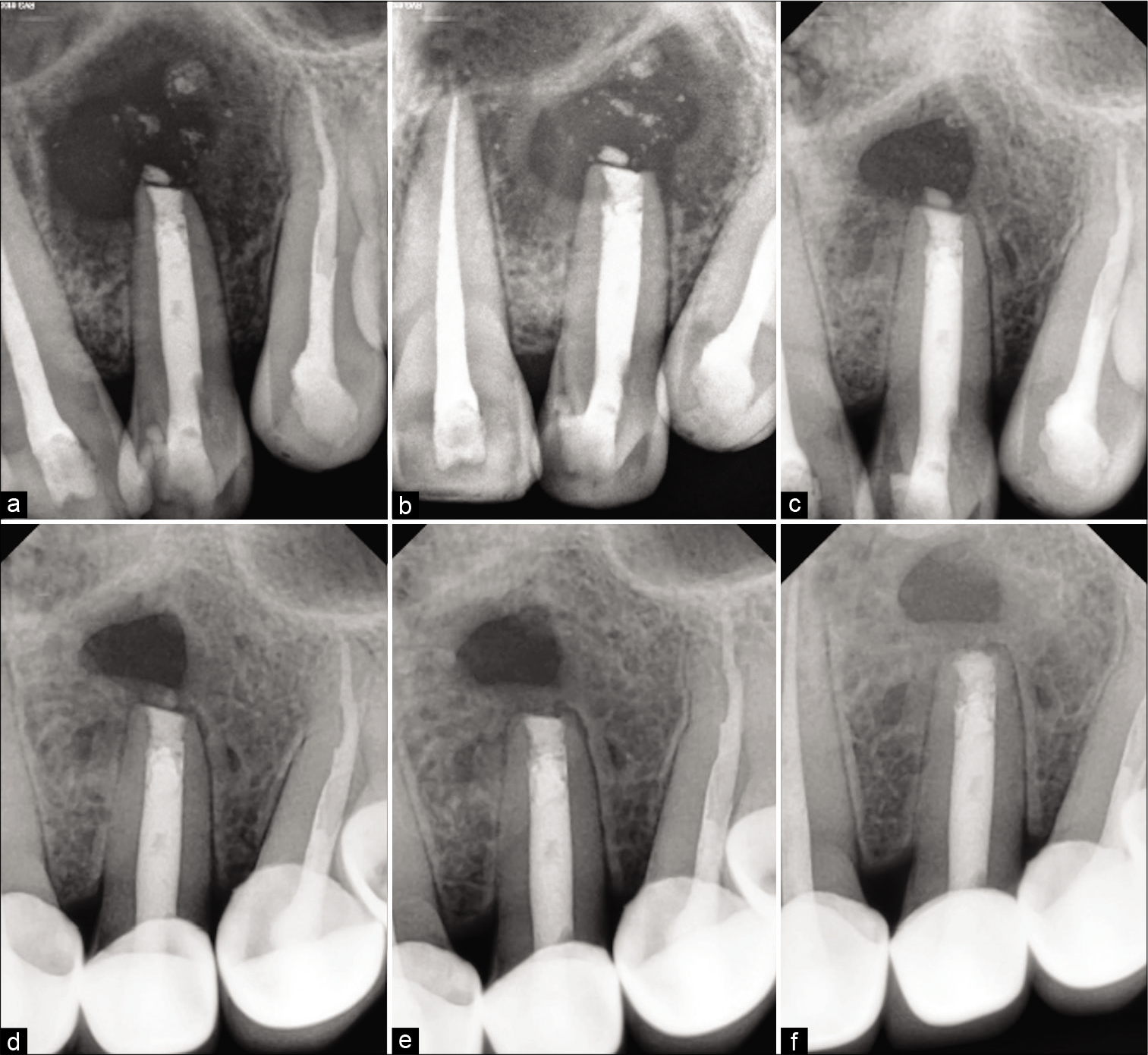
- Follow-up radiographs (a) 3 months postoperative radiograph showing the evidence of bone formation (b) 6 months postoperative radiograph showing the array of trabecular bone formation (c) 1-year post-operative radiograph showing the complete trabecular bone formation with the well-defined radiolucent area associated with the lateral incisor (d) 2-year post-operative radiograph showing the bone formed between the radiolucent area and associated with the lateral incisor with nearly intact lamina dura (e) 3-year post-operative radiograph showing 1.5–2 mm distance within the radiolucent area and associated with the lateral incisor (f) 4-year post-operative radiograph showing the intact lamina dura with 22, 21, and 23 along with cementum formation along the apex of 22. Approximately, 2 mm of trabecular bone formed between the radiolucent area and associated with the lateral incisor suggestive of intraosseous fibrous scar formation.
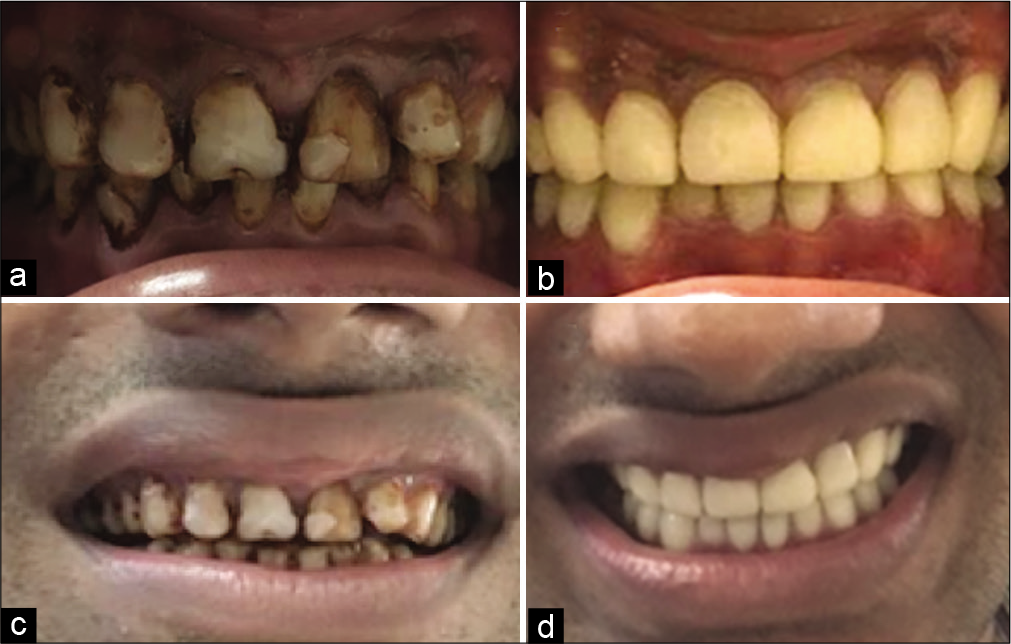
- (a) Frontally centered pre-operative intra-oral photograph showing generalized moderate to severe fluorosis stains (b) frontally centered post-operative intra-oral photograph showing aesthetic rehabilitation with full-coverage crowns (c) Face-frontal (smiling) pre-operative photograph (d) Face-frontal (smiling) post-operative photograph.
DISCUSSION
Term dens invaginatus was introduced by Hallet (1953) to define this tooth developmental anomaly in which enamel is centrally located and the dentine is located peripherally due to the invagination.[1] The enamel is very thin and less mineralized or sometimes even absent in these invaginations. Various studies have reported interruptions formed on the surface of the tooth in form of a fissure or groove especially on the palatal aspect, facilitate the penetration of the irritants directly into the pulp, which might be the reason for increased risk go pulpal and periodontal pathosis. Invagination may contain concurrent tissues of dental papilla which when get necrosed becomes nutrition for the bacteria and further worsen the condition.[13]
In dens invaginatus cases, some areas of afibrillar cementum organic material are derived from epithelial cells of Malassez (Kodaka 2002) which is responsible for causing various pathologies.[14] Thus, the cell rest of malassez which are a known causative factor for the formation of an inflammatory apical cyst are thought to be substantially present in dens invaginatus cases. These cell rest of Malassez which might be present due to the dens invaginatus, also interfere with the Osteoid bone formation during the healing process following endodontic surgery. Cytokines are released which cause rapidly progressive defensive fibro production and scar formation. Thus, osteoblasts cannot differentiate into bone completely, which may be a probable cause for incomplete healing with intraosseous fibrous scar formation.[15]
In the periapical healing with intraosseous fibrous scar formation, an isolated rarefaction remains in the bone while in the bone surrounding the rarefaction regeneration continues and the lamina dura may also be formed around the apex. A close correlation was found between the histologic demonstration of scar tissue and incomplete healing where a rarefaction could be seen. In the study by Rud et al., in 90% cases of incomplete healing, radiographic and histologic evaluators were able to find of the fibrous scar tissue. In 61% of the cases, moderate to severe periapical inflammation was reported with incomplete healing. They further mentioned that the intraosseous fibrous scar tissue has no clinical importance, until it is not inflamed.[9] Hence, no surgical intervention is required until it is asymptomatic, can be presumed. Hence, a correct diagnosis and knowledge about this developmental anomaly can help a clinician to get over such a dilemma of surgical re-intervention turmoil and its consequences.
In an endodontic surgical site, osteogenesis is evident in about 6 days after surgery. Three to four weeks postoperatively, an excisional osseous wound is 75–80% occupied with trabecular bone which is encircled by extremely active osteoid and osteoblastic cells. The osseous defect is typically filled with trabecular porous bone by around 16 weeks of surgery.[12] Within 10–12 days after root-end resection cementogenesis starts. Newly formed PDL fibers functionally realign and rearrange themselves extending from the recently formed cementum into the woven trabecular bone. This takes place about 8 weeks after surgery.[16]
Hence, in the above case, a 4-year of follow-up without any clinical signs and symptoms, and persistent radiographic rarefaction with healthy trabecular bone surrounding the radiolucency along with intact lamina dura with the involved tooth took to present diagnosis of the incomplete healing with intraosseous fibrous scar formation.
Another probable reason for incomplete healing and intraosseous fibrous scar formation can be the use of (nonsteroidal anti-inflammatory drugs) NSAIDs postoperatively. NSAID inhibits the enzyme cyclooxygenase, which is associated with the synthesis of prostaglandins which regulate osteoblast proliferation and differentiated functions required for bone healing.[17,18] These drugs also have been shown to retard the fracture healing process and to affect bone formation adversely in animals and humans.[19]
The radiographic feature of the intraosseous fibrous scar is very similar to those of the residual cyst. However, there is always an associated missing tooth history present with residual cyst. Residual dental cysts are often discovered as accidental findings on routine radiographs.[12] Residual cysts are radicular cysts that remain or form after the responsible tooth has been extracted, the cyst remains in the bone tissue without having been excised.[20] Very less information is there to differentiate clinically and radiographically between an asymptomatic non-expanding residual cyst and intraosseous fibrous scar except for the hypothesis that residual cyst is always associated with extracted tooth site.
To the best of our best knowledge, no such case has been reported regarding an asymptomatic endodontically treated Type II dens invaginatus in lateral incisor with intact lamina dura, healthy trabecular bone, and normal periodontal space healed with the intraosseous scar formation associated with persistent well-defined rarefaction associated with a healing previously surgically enucleated site. The probable causes, diagnosis, treatment line, prognosis and implications of dens invaginatus, and intraosseous fibrous scar were explained in the current case report to reduce the stage dilemma of a clinician in such case scenarios.
CONCLUSION
Treating the dens invaginatus presents a myriad of complexities. To identify such developmental anomalies, cone beam computed tomography is deemed necessary to understand the extent and complexities of the case. In addition, incomplete periradicular healing after surgical management presenting as intraosseous fibrous scar with a persistent well-defined radiolucency mimicking a residual cyst radiographically still remains an area for further clinical and observational researches.
Acknowledgment
The authors acknowledge Dr. Manjushree Bhandari, Chairman, Sri Aurobindo College of Dentistry, SAIMS, and Dr. Vinod Bhandari, Founder Chairman, SAIMS for their guidance and support toward this manuscript.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Dens invaginatus. Part 1: Classification, prevalence and aetiology. Int Endod J. 2008;41:1123-36.
- [CrossRef] [PubMed] [Google Scholar]
- Dens invaginatus: A series of case reports. J Oral Res Rev. 2018;10:20-3.
- [CrossRef] [Google Scholar]
- Dens invaginatus: A retrospective study of prophylactic invagination treatment. Int J Paediatr Dent. 2001;11:927.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of dens invaginatus in North Indian population. Oral Maxillofac Pathol J. 2013;4:282-8.
- [Google Scholar]
- The epithelial cell rests of Malassez-a role in periodontal regeneration? J Periodontal Res. 2006;41:245-52.
- [CrossRef] [PubMed] [Google Scholar]
- Proliferation of epithelial cell rests, formation of apical cysts, and regression of apical cysts after periapical wound healing. J Endod. 2007;33:908-16.
- [CrossRef] [PubMed] [Google Scholar]
- The integrated processes of hard tissue regeneration with special emphasis on fracture healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:594.
- [CrossRef] [Google Scholar]
- Radiographic criteria for the assessment of healing after endodontic surgery. Int J Oral Surg. 1972;1:195-214.
- [CrossRef] [Google Scholar]
- Correlation between histology and radiography in the assessment of healing after endodontic surgery. Int J Oral Surg. 1972;1:161-73.
- [CrossRef] [Google Scholar]
- A follow-up study of 1, 000 cases treated by endodontic surgery. Int J Oral Surg. 1972;1:215-28.
- [CrossRef] [Google Scholar]
- Residual cysts: A brief literature review. Int J Med Dent Sci. 2016;5:1341-6.
- [CrossRef] [Google Scholar]
- Scanning electron microscopy and energy-dispersive X-ray microanalysis studies of afibrillar cementum and cementicle-like structures in human teeth. J Electron Microsc (Tokyo). 2002;51:327-35.
- [CrossRef] [PubMed] [Google Scholar]
- Etiopathogenesis of post-endodontic periapical scar formation. Dent Hypotheses. 2012;3:5-15.
- [CrossRef] [Google Scholar]
- Periodontal regeneration: Root surface demineralization. Periodontol 2000. 1993;1:54-68.
- [CrossRef] [Google Scholar]
- Bone cell biology: New approaches and unanswered questions. J Bone Miner Res. 1993;8:S457-65.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of nonsteroidal anti-inflammatory drugs and prostaglandins on osteoblastic functions. Biochem Pharmacol. 1999;58:983-90.
- [CrossRef] [Google Scholar]
- Effects of indomethacin and local prostaglandin E2 on fracture healing in rabbits. Dan Med Bull. 1996;43:317-29.
- [Google Scholar]






